Challenging the Status Quo
Increase efficiency & quality in pharmaceutical production, powered by a modular validated platform for continuous manufacturing (CM) .
Proud to be working with
How we push boundaries

Flexibility
Designed as standard building blocks, ContiUnity® adapts to your unique requirements, offering a perfect fit for numerous setups and processes.

Time to market
By selecting pre-validated ContiUnity® modules, you can bypass the design phase and configure your process plant quickly and efficiently.

Risk reduction
ContiUnity® is a multipurpose solution that can be quickly adapted to changing situations, eliminating the need for additional capital investments, resulting in cost reduction.

Sustainable
The intelligent modular design reduces floorspace by 70% compared to traditional batch facilities, with modules being reusable in future processes as well.

The new standard for the pharmaceutical industry
Developed in collaboration with leading pharmaceutical companies, ContiUnity® is the only pre-validated modular system for the continuous manufacturing of pharmaceutical active ingredients. It offers a complete solution that integrates modular hardware with intuitive process control strategies, tailored to your needs.
Project Approach
PROCESS TRANSLATION
Together with you, Zeton will translate your vision into the ContiUnity® architecture, tailored precisely to your needs.

MODULE SELECTION
Modules are selected from our library of pre-engineered units. If a custom module is required for a process, this can be designed in parallel.
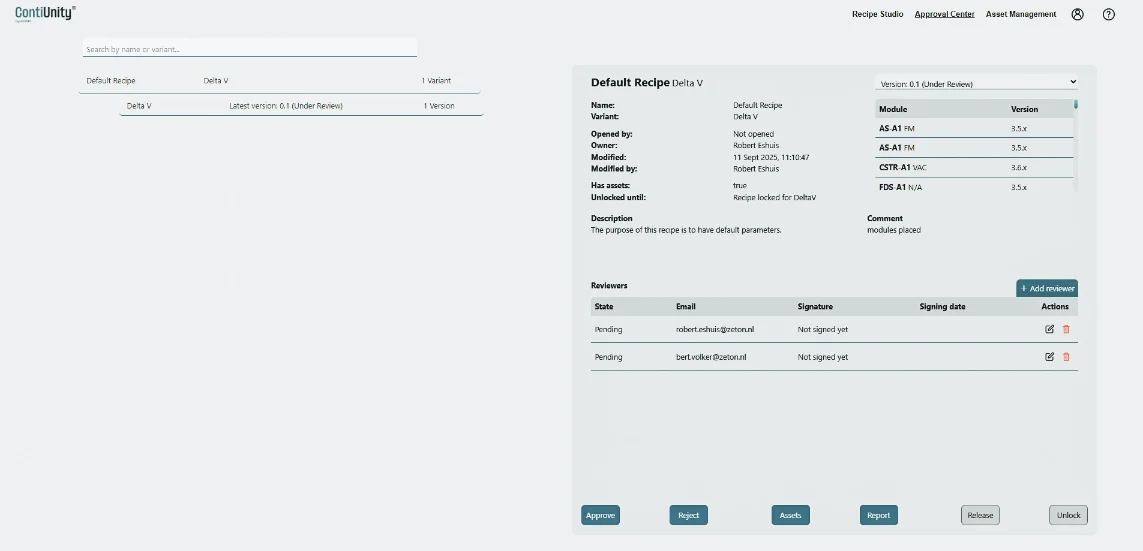
ASSEMBLY & TESTING
At Zeton, we efficiently and skillfully assemble the complete system and subject it to rigorous IQ and OQ testing to ensure top quality.


PACKAGING & TRANSPORT
After successfully passing all tests, we will pack and ship the system. Limited disassembly is required due to ContiUnity®'s modularity.

ON SITE TESTING
With pre-validated modules and the system fully tested at Zeton, only limited Site Acceptance Testing is required before system handover.

Latest insights
Successful MTP Workshop With One Of Our Clients

Zeton BV officially accredited for the French Crédit d’Impôt Recherche (CIR) program












